The study reported significantly prolonged progression-free survival and a 44% improvement in overall survival. Recently, CAR-T has also shown great potential in treating autoimmune diseases such as systemic lupus erythematosus and multiple sclerosis.
However, CAR-T development has long faced a persistent challenge: each new CAR targeting a novel tumor antigen requires the iterative development of a fully validated detection reagent to confirm CAR surface expression. This process is not only costly and time-consuming but may also introduce data bias due to insufficient antibody specificity, significantly delaying project timelines.
To address the challenge, Sino Biological has developed anti-whitlow 218 linker rabbit monoclonal antibodies, which specifically binds to the whitlow 218 linker sequence (GSTSGSGKPGSGEGSTKG). The antibodies are independent of antigen specificity, making it applicable across multiple CAR constructs and target antigens.
What is CAR Structure?
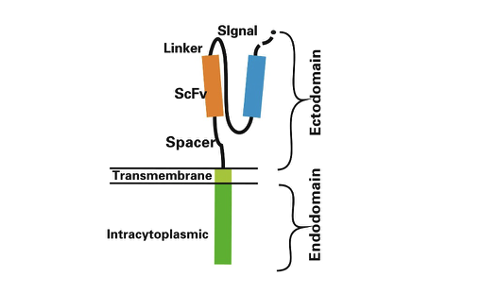
A CAR (Chimeric Antigen Receptor) is a genetically engineered receptor that combines the antigen-binding ability of an antibody with T-cell activation capabilities. The CAR structure comprises four main components:
1) Extracellular Antigen-Binding Domain: The core targeting region, typically a single-chain variable fragment (scFv), designed to recognize specific tumor-associated antigens with high specificity.
2) Hinge Domain: Spans the extracellular antigen-binding domain and the transmembrane domain, providing flexibility and supporting proper antigen-binding conformation.
3) Transmembrane Domain: Anchors the CAR structure into the T-cell membrane, ensuring stability and functionality.
4) Intracellular Signaling Domain: Activates the T cell upon antigen engagement. It usually contains the CD3ζ chain or CD3ε derived from the T-cell receptor, plus costimulatory domains such as CD28 or 4-1BB to enhance persistence and anti-tumor activity.
Why Measuring CAR Expression is Important?
The CAR positive rate is a critical metric for evaluating CAR-T product potency, directly influencing target recognition and cytotoxic efficacy. Monitoring CAR expression in peripheral blood or tissues post-infusion provides insights into in vivo expansion and persistence, informing treatment response, durability, and potential relapse risk. Dynamic CAR expression monitoring also helps assess immunogenicity and safety. Thus, a sensitive, reliable, and universal CAR detection system is vital for developing and evaluating CAR-T cell therapies.
Currently, approved CAR-T products target CD19 or BCMA, making flow cytometry with recombinant BCMA or CD19 protein the standard detection method. Although highly specific, this approach is limited to single targets and is unsuitable for CAR-T screening and preclinical development.
Several CAR detection methods have been developed, including Protein L (which binds immunoglobulins) or Fc-binding polyclonal antibodies. Protein L binds only to kappa light chains and fails to detect CARs containing lambda chains. Polyclonal antibodies may cross-react with patient IgG, causing false positives. Anti-idiotype antibodies offer high specificity and sensitivity by targeting unique idiotopes in the CAR’s antigen-binding region, but they are challenging to develop and produce.



No Comments
Leave a comment Cancel