ITM Isotope Technologies Munich SE has raised €188m ito boost radiopharma pipeline, contract manufacturing cpacity, and launch ITM-11.
Radiopharma conjugate specialist Isotope Technologies Munich SE has raised €188m in an equity financing led by globally active investor Temasek. BlackRock, Qatar Investment Authority (QIA), ATHOS and Carbyne participated in the financing that will be used to boost the company’s alpha- and beta-emitting radioisotope pipeline of radiopharmaceuticals, enable an expansion of manufacturing capabilities for Lutetium-177 (177Lu) and Actinium-225 (225Ac) for customers, and prepare the market launch of the company’s Phase III orphan drug ITM-11/177Lu-edotreotide for the treatment of gastroenteropancreatic neuroendocrine tumours (GEP-NETs).
Steffen Schuster, CEO of ITM, commented: “We have reached key milestones since our last financing round including advancing our pipeline with novel targets in multiple oncology indications, opening our NOVA facility, launching Actineer with CNL for the joint production of Actinium-225 and entering several high-value supply agreements for Lutetium-177.” _
ITM uses its medical radioisotopes for the diagnosis and targeted treatment of cancer. ITM-11 (177Lu-edotreotide) is being evaluated for the treatment of gastroenteropancreatic neuroendocrine tumors (GEP-NETs) in two pivotal open-label Phase III studies, COMPETE for grade 1 and grade 2 GEP-NETs and COMPOSE for grade 2 and grade 3 GEP-NETs.
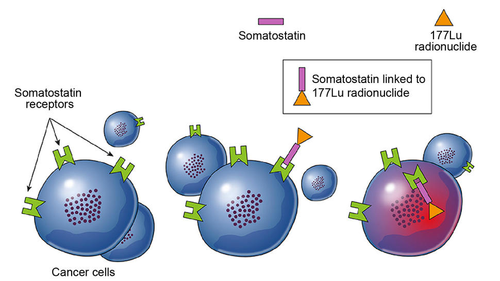
In October 2022, the US FDA granted fast track designation to ITM-11 as a treatment for patients with GEP-NETs. The agent consists of lutetium-177 and the targeting molecule edotreotide, which is a synthetic formulation of peptide hormone somatostatin.
Radiopharma therapy is a class of cancer therapeutics, which seeks to deliver radiation directly to the tumour while minimising radiation exposure to normal tissue. Targeted radiopharmaceuticals are created by linking a therapeutic radioisotope such as Lutetium-177 or Actinium-225 to a targeting peptide, antibody, or small molecule that homes in at tumour-specific receptors on the tumour cell surface.
According to market analyses by Markets & Markets, radiopharmaceuticals generated global sales of US$5.5bn in 2023. The market is forecasted to grow to US$9.4bn by 2028. Radiopharmaceuticals have recently been the subject of increasing major investments by pharmaceutical companies. In May, Swiss-based Novartis AG acquired US-based Mariana Oncology for US$1bn. Shortly afterwards, Bayer AG signed a collaboration agreement with UK-based Bicycle Therapeutics plc, which could bring the British company US$1.7bn if its cyclic peptides (Bicycles) prove to be able to selectively deliver radioisotopes close enough to tumours. Like other targeted drug classes, conjugate strategies such as ADCs also suffer from the fact that more than 80% of tumours are “cold”, i.e. carry little or no antigens on their surface. These are often actively secreted into the blood (antigen shedding) in order to evade targeted immune responses.
177Lu-peptide conjugates are not a novelty. The US FDA approved the first 177Lu-somatostatin-targeting peptide 177Lu dotatate (Luthathera) to treat gastroenteropancreatic neuroendocrine tumours back in 2018, one year after the EU approved the radiopharmaceutical developed by French Advanced Accelerator Applications SA, which is a 177Lu customer of ITM. The company had been taken over in 2017 by Novartis AG for US$3.9bn due to positive results of the NETTER-1 study: Patients who received Lu 177-dotatate plus octreotide lived almost three times as long without their cancer getting worse as those who received octreotide alone—a median of nearly 23 months, versus 8.5 months. The publication of corresponding post-Phase I data on ITM-11 is eagerly awaited.
According to the American Society of Clinical Oncology, (ASCO) the overall five-year relative survival rate for NETs in the GI tract is 94%. For GI NETs that have not spread beyond the area of origin, the five-year relative survival rate is 97%. According to experts from University Hospital Düsseldorf, 10 year survival for metastasized GEP-NETs is around 50%. Radiotherapy of GEP-Nets along with chemotherpy and somatostatin analogs or mTOR blockers are second-line therapies used after tumour surgery.














No Comments
Leave a comment Cancel