Diagnostics company SphingoTec GmbH (Hennigsdorf, Germany) has successful closed a €5m Series C financing round led by Think.Health Ventures, with support from existing investors Brandenburg Kapital, HBM Healthcare Investments, NRW.BANK, and Wellington Partners. The raised capital will allow the company to reach profitability through partnerships and licence deals of its kidney function test penKid and the prognostic shock biomarker bioADM. “This funding will support our global expansion, including entry into the US market, and drive the commercialisation of our innovative biomarkers for critical care,” a Sphingotec spokeswoman told European Biotechnology. According to Sphingotec, lead investor Think.Health Ventures will provide support to the SphingoTec management team through its extensive network in clinics and laboratories.
Dr. Florian Kainzinger, Managing Partner and Founder of Think.Health Ventures, commented, “We are pleased to support SphingoTec at this exciting stage of the company’s development. With its developed and commercialized lead product penKid, SphingoTec is well-positioned for global expansion through out-licensing to key players in the diagnostic industry.”
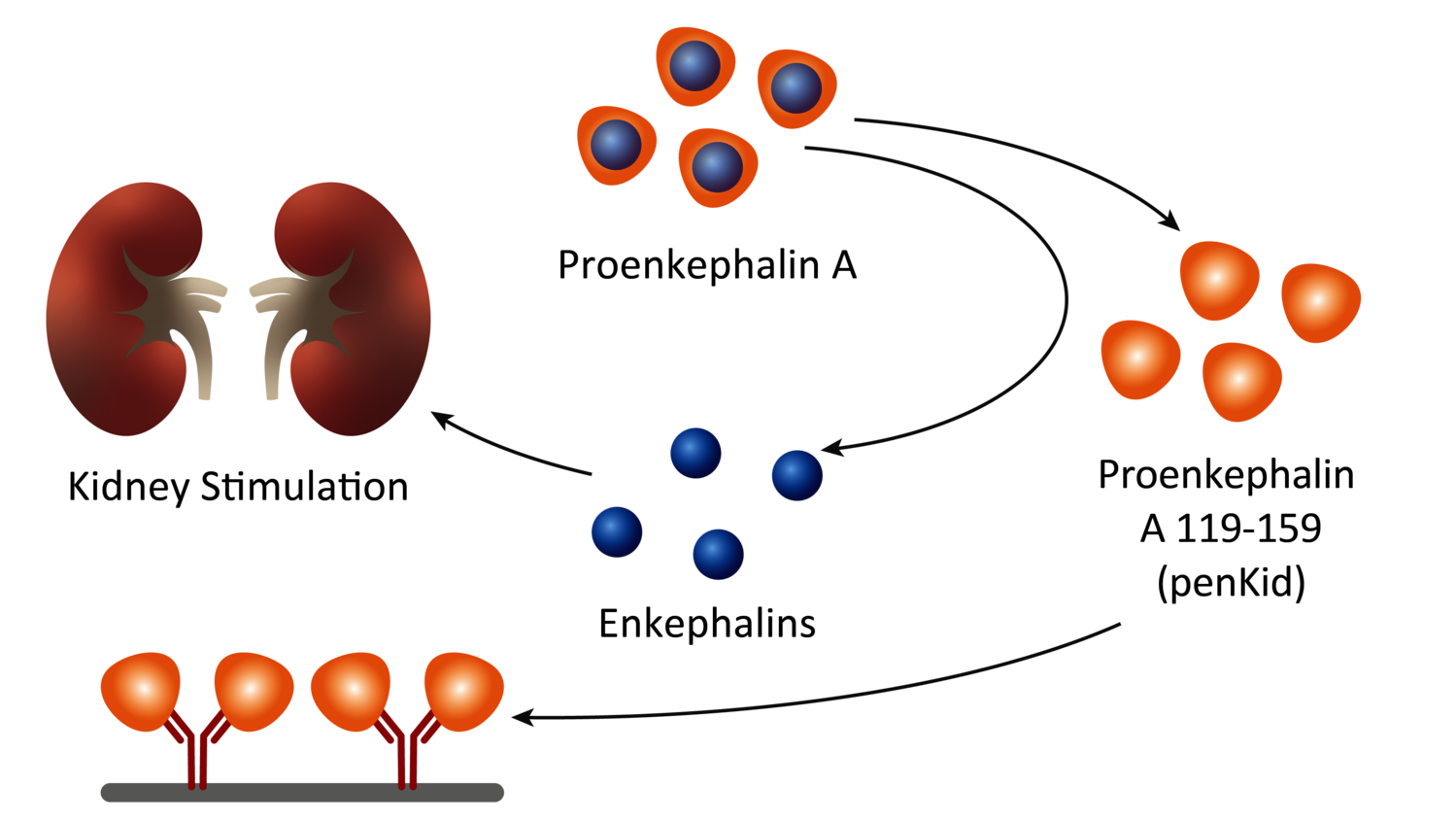
SphingoTec focuses on translational medicine, bringing innovations to frontline healthcare by discovering biomarkers, developing IVD assays, and making them available on different diagnostic platforms. The biomarker portfolio includes Proenkephalin A 119-159 (penKid), a biomarker for the assessment of kidney function, and bioactive Adrenomedullin 1-52 (bio-ADM), a biomarker for the assessment of endothelial function. bioADM is used by sepsis drug maker Adrenomed GmbH for patient stratification. Adrenomed recently got FDA fast track designation for its Phase II antibody enibarcimab (formerly adrecizumab) that restores endothel integrity.
According to rumours, Sphingotec is shortly before to close a two-digit million US dollars deal with a not yet discloded diagnostics big shot for penKid. Last year, Sphingotec widened a deal with South-Korean BoditecMed Inc to distribute its penKid assay together with Boditec’s automated AFIAS platform.
Penkid is a proenkephalin A fragment that is stable in the blood and a surrogate marker for the peptide hormone enkephalin, which is rapidly degraded and therefore previously unmeasurable, and is released in the event of acute kidney damage. The fact that high penKid levels indicate acute renal dysfunction has been proven by chief intensive care physicians at the University Hospitals of Aachen and Heidelberg, who have been routinely validating the biomarker test in pilot projects since 2020 and 2022 respectively.
Around 20% of patients in intensive care units, an estimated 13 million worldwide, develop acute kidney injury, which is often diagnosed too late. The new biomarker enables timely intervention in cases of cardiogenic or septic shock, for example, where kidney failure is a common cause of death.
Deborah Bergmann, Managing Director and CEO, stated, “There is a substantial demand for more advancements in critical care diagnostics. Our scientifically and clinically validated innovations have the potential to improve the management of critically ill patients. With this investment, we will focus on further out-licensing our biomarkers to translate scientific advancements into routine clinical practice, ultimately supporting clinicians in improving patient outcomes.”
Bioactive Adrenomedullin 1-52 (bio-ADM) is a dynamic blood biomarker for the real-time assessment of endothelial function. The endothelium contributes to blood pressure and separates blood from the surrounding tissue. Elevated bio-ADM blood levels predict both blood pressure drop, resulting in shock and leaky blood vessels, leading to edema formation. Early identification of an imbalance in endothelial function allows for the prediction of vasopressor demand in critically ill patients and aids in the identification of residual congestion in acute heart failure patients.



















No Comments
Leave a comment Cancel