The therapeutic landscape for relapsed or refractory (R/R) B-cell non-Hodgkin lymphoma (B-NHL) is evolving toward targeted T-cell based immunotherapies, including CD19-targeted CAR-T and CD3xCD20 T-cell engaging (TCE) bispecific
antibodies. Yet, there remains an unmet medical need for patients who are refractory to, or ineligible for these treatments. Leveraging natural killer NK cells emerges as a promising strategy in hematological malignancies.
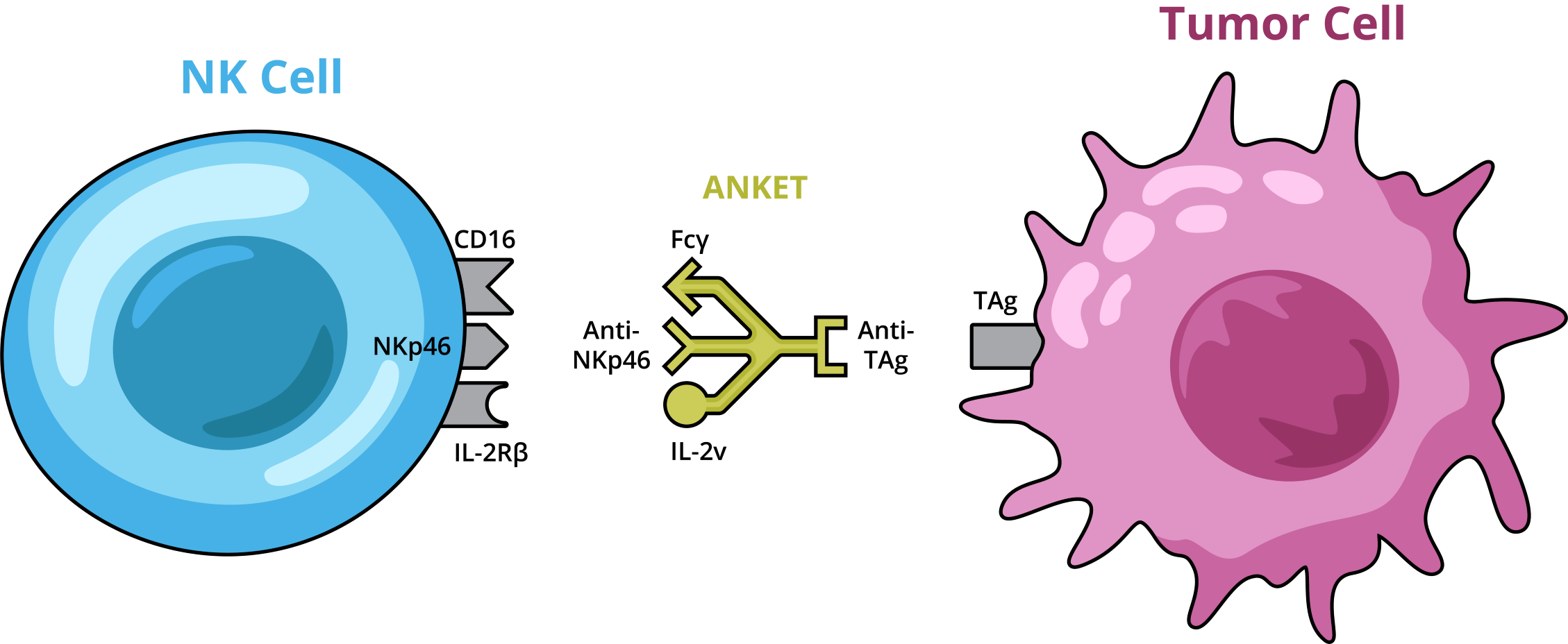
In late November Innate Pharma SA published preclinical results of IPH6501, the company’s first tetra-specific NK cell engager to engage activating receptors (NKp46 and CD16), a tumour associated antigen (CD20) and an interleukin-2 receptor (via an IL-2 variant, IL-2v) via a single molecule (10.1126/sciimmunol.adp3720), using Innate’s proprietary multispecific antibody format ANKET (Antibody-based NK cell Engager Therapeutics).
IPH6501 was bound to both, cancerous B cells and natural killer (NK) cells, activating the immune cells of the innate immune system to target blood cancer cells. The treatment showed higher efficacy and lower toxicity than T cell engager-based existing B-NHL therapies in mouse models and non-human primates, and is currently undergoing Phase I evaluation in relapsed or unresponsive human B-NHL patients.
Novel NHL treatments such as T cell engagers (TCEs) have shown clinical promise but often cause serious toxicities. Recent research has suggested that NK cell-based treatments may offer effective and safer alternatives to some T cell therapies. Building on these findings, Olivier Demaria and colleagues developed IPH6501, that can activate NK cells through three different receptors while also targeting the tumour-associated antigen CD20 on B-NHL tumours. Notably, the molecule was designed to include an IL-2 variant that could trigger NK cell proliferation without activating regulatory T cells (Tregs) but did not show IL-2-related side effects – a common phenomenon in TCE treatments that can cause therapy resistance.
The team headed by CSO Eric Vivier also produced a variant of the molecule, named CD20-NKCE-IL2v, with a key change in one domain to enable testing in mice. IPH6501 promoted NK cell activation in vitro, and exhibited higher anti-tumour efficacy and lower toxicity than CD20-targeting TCEs. Demaria et al. demonstrated that CD20-NKCE-IL2v and IPH6501 elicited NK cell expansion and migration to tumour sites in mouse models and non-human primates, respectively. IPH6501 also induced proliferation and efficient anti-tumour activity in NK cell samples from relapsed or unresponsive B-NHL patients.
Furthermore, IPH6501 could activate NK cells to target CD20-negative tumour cells, indicating its broad therapeutic potential against varied lymphomas. IPH6501 showed strong antitumour efficacy in a tumour model resistant to rituximab and obinutuzumab and post-Car-T cell therapy.
Currently, IPH6501 is in a Phase I/II dose-escalation trial with a study design presented at this year’s ASCO conference.


















No Comments
Leave a comment Cancel